Shop
Filter by price
Stock status
Showing 229–240 of 1124 results
Cellular Blankets
Cellular Blankets
Cellular Blankets are lightweight, breathable hospital blankets made from soft cotton or polyester materials, designed to provide warmth and comfort while allowing excellent air circulation. Their cellular (honeycomb) structure helps regulate body temperature, making them ideal for patients in hospitals, clinics, and nursing homes.
Cellular Blankets
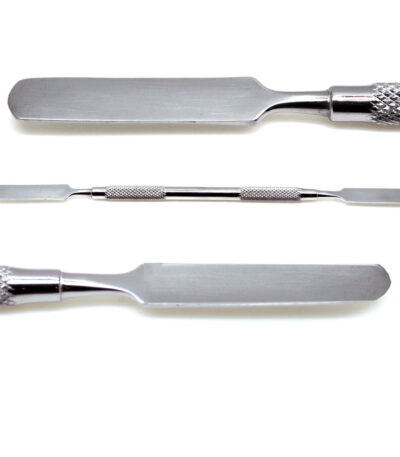
Cement Spatula
Centrifuge 6 Tubes
Centrifuge 8 Tubes
Cerebral Palsy Child Walker
Cerebral Palsy Wheelchair (Kenya)
Celebral Palsy Wheelchair,
Celebral Palsy is a special condition that is caused by abnormal development of the brain or damage to the developing brain that affects a child’s ability to control his or her muscles.
Made from high quality aluminium.
It can reclining high back.
it seat can be tilted.
Has a detachable armrest and elevating legrest.
Cervical Hot Pack
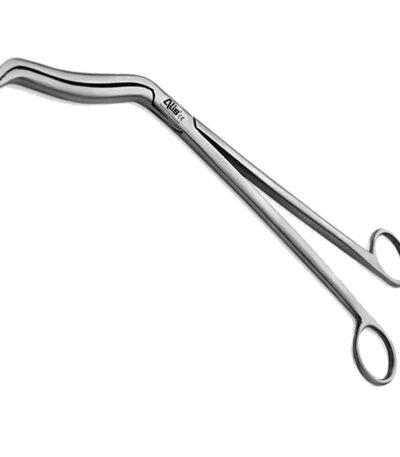
Cheatle Forceps
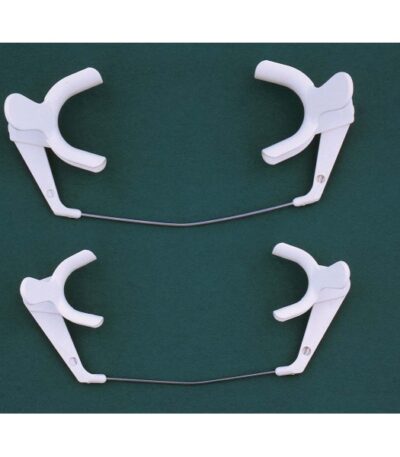
Cheek Retractor (Adult / Paediatric)
A Cheek Retractor, also known as a Mouth Retractor or Lip and Cheek Expander, is a dental instrument used to pull and hold the cheeks and lips away from the teeth and gums during dental or orthodontic procedures. This provides the dentist or hygienist with clear visibility and easy access to the working area inside the mouth.